
Scalable Cleanroom Systems | Insights from Lewis Irish
An Interview with Lewis Irish, Sales Director at Monmouth Scientific.
In this interview, News Medical-Life Sciences speaks with Lewis Irish, Sales Director at Monmouth Scientific, about the design and benefits of modular cleanrooms and how they support scalable, compliant environments across biomedical, life sciences, and manufacturing industries.
Commercial and Market Insights from the Cleanroom Sector.
Can you please introduce yourself and your role at Monmouth Scientific?
My name is Lewis Irish, and I’m the Sales Director at Monmouth Scientific. I oversee our sales operations, working closely with clients across a wide range of industries to deliver modular cleanroom solutions that meet their evolving needs. My role involves ensuring that our cleanrooms are tailored to specific technical and regulatory requirements and helping clients futureproof their facilities through scalable and adaptable designs.
Could you provide an overview of Monmouth Scientific’s Modular Cleanrooms and what sets them apart in the market for controlled environments?
At Monmouth Scientific, we design and supply Modular Cleanrooms for various sectors that require controlled environments. What sets our systems apart is their modularity, scalability, and adaptability. Unlike traditional fixed cleanrooms, our modular units are designed for quick reconfiguration and expansion. This allows clients to adapt to changing operational demands without the time, cost, and disruption associated with conventional builds.
Our cleanrooms are designed to meet stringent ISO standards and can be seamlessly integrated into complex facilities. This flexibility makes them ideal for organisations seeking high-performance environments without compromising compliance or operational flow.
The cleanroom systems are described as modular, expandable, and adaptable. Can you elaborate on how this design philosophy benefits aerospace, electronics, and industrial manufacturing industries?
Industries such as aerospace, electronics, and manufacturing operate in fast-paced environments. They often need to adjust for new production lines, evolving regulations, or upgraded equipment. Our modular cleanrooms are designed explicitly for that purpose.
Clients can reconfigure or expand their cleanroom setup without interrupting production. For example, aerospace manufacturers can install new testing rigs, electronics companies can increase production capacity, and industrial firms can switch to new product lines, all while maintaining contamination control. It is about enabling innovation and agility without being restricted by infrastructure.

The ability to rapidly install and reconfigure cleanrooms is a significant advantage. What engineering and design strategies have been implemented to enable fast deployment and operational flexibility?
We achieve this via pre-engineered panels, integrated service channels, and standardised connection systems. These components enable fast assembly, often in a fraction of the time required for traditional construction.
Our cleanrooms are also designed to include relocatable walls, removable ceiling grids, and modular lighting, HVAC, and filtration systems. These built-in design features enable easy adaptation of the cleanroom layout as operational needs evolve, with minimal disruption and downtime.
Scalability is clearly a key feature of the cleanroom solutions. Could you discuss how the systems accommodate large-scale cleanroom facilities without compromising performance or compliance?
Scalability is at the heart of our modular design. We use repeatable, high-quality components that can be extended as needed to create facilities covering hundreds of square metres. These expansions do not compromise airflow management or ISO classification.
Our systems also include integrated monitoring solutions to ensure ongoing regulatory compliance across the entire space. Whether the client starts small or builds large from day one, performance remains consistent and verifiable.
Many industries face evolving operational needs. How do the cleanrooms support organisations as the scale up, change layouts, or introduce new equipment?
We design with flexibility and long-term use in mind, and cleanrooms can be reconfigured internally. Walls, ceilings, and services can all be moved or expanded. As operations scale, production lines change, or larger equipment is introduced, the cleanroom can adapt with minimal cost and no major reconstruction.
This means our clients can maintain ISO-class compliance throughout operational changes, avoiding costly rebuilds or lengthy downtimes.
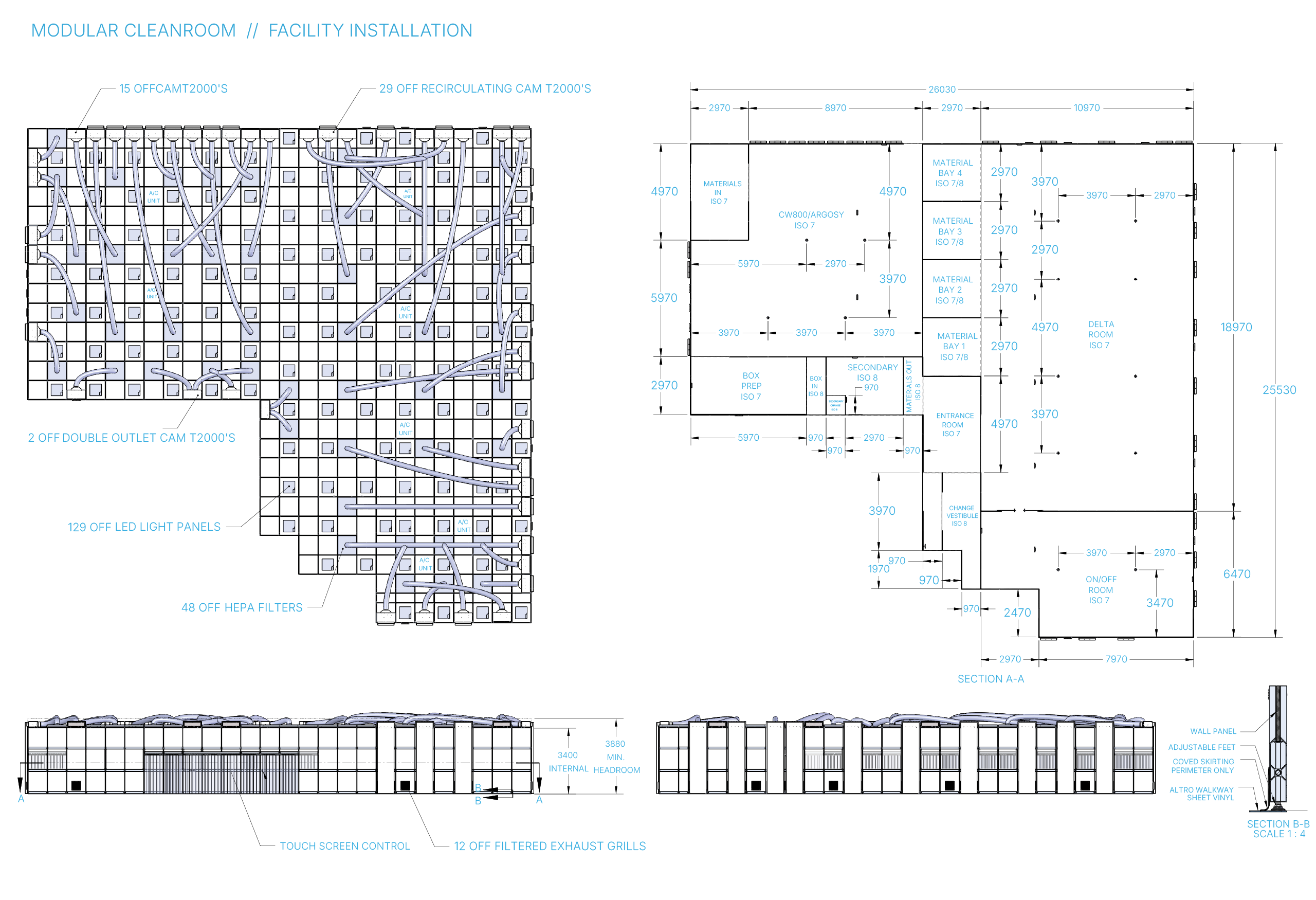
How does the modular cleanroom design address the unique challenges of biomedical and life sciences sectors, particularly in terms of contamination control and regulatory compliance?
In biomedical and life sciences, regulatory compliance and contamination control are non-negotiable. Our cleanrooms are designed to meet strict ISO classifications, allowing for sterile and particle-controlled environments that support sensitive research and production.
A notable example is our collaboration with WOMED, where we designed modular, ISO-classified cleanrooms to support essential processes while also accommodating future expansion. Our approach provides a clean environment and one that evolves with the organisation, without compromising safety or standards.
In terms of long-term investment, how does the reusability and relocatability of the cleanroom systems offer a more sustainable or cost-effective solution for clients?
Our cleanrooms offer long-term value because they are not fixed assets. Panels, service channels, and modular elements can be reused when reconfiguring or relocating the space, which reduces material waste and capital expenditure.
From a sustainability perspective, this significantly reduces environmental impact compared to traditional construction methods. Operational flexibility paired with resource efficiency is a win for both budgets and corporate sustainability goals.
What feedback has been received from clients who have deployed large-scale cleanroom facilities using the modular systems, particularly regarding installation timelines, ongoing flexibility, or ROI?
The feedback is overwhelmingly positive. Clients regularly cite the speed of installation and flexibility as major advantages. We have helped clients deploy large-scale cleanrooms in a fraction of the time it would take using traditional methods, which in turn accelerates their time-to-market.
Many also appreciate how easily the space can be modified in the future. As a result, their return on investment is often achieved much sooner, particularly when the modular design facilitates growth without needing costly extensions or downtime.
Are there any recent installations or case studies that effectively demonstrate the scale and adaptability of your cleanroom technology?
Yes. One standout case is our project with ZeroAvia. In 2022, we installed a 14m x 5m ISO 8 Modular Cleanroom at their UK site. As their operations scaled, they needed more cleanroom space. We were able to deliver a seamless expansion, a 14m x 9m extension, bringing the total space to 14m x 19m.
This project is an excellent example of how our modular systems can grow with the client’s needs while maintaining performance, compliance, and operational continuity.

About Lewis Irish
Lewis Irish is the Sales Director at Monmouth Scientific. With more than a decade of experience in the cleanroom and laboratory containment industry, Lewis has helped guide the adoption of scalable cleanroom technologies across the life sciences, manufacturing, and aerospace sectors.
Before joining Monmouth Scientific, Lewis held several technical and commercial roles that spanned project management and cleanroom consultancy.
At Monmouth Scientific, he plays a pivotal role in aligning clients’ operational goals with flexible, compliant cleanroom solutions, whether for research, production, or advanced manufacturing. His work reflects a deep understanding of regulatory environments, as well as the need for adaptable infrastructure in rapidly evolving industries.
Trusted by Leading Facilities.
Monmouth Scientific. 2025. Modular cleanrooms: Enhancing flexibility and compliance in controlled environments. News-Medical, viewed 25 November 2025, Modular cleanrooms: Enhancing flexibility and compliance





